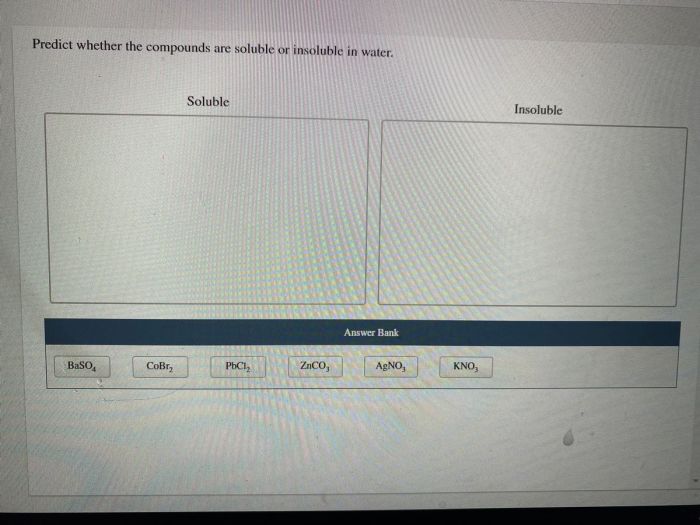
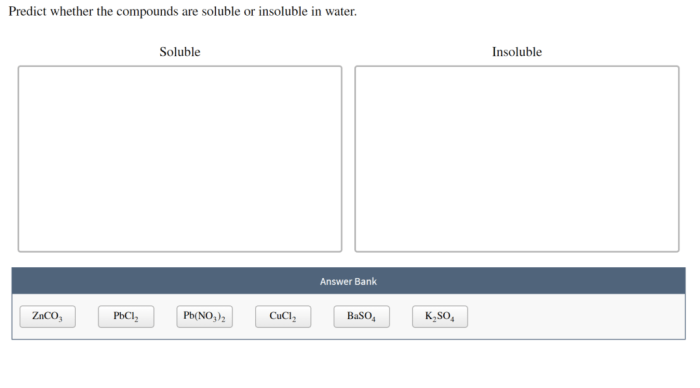
Predict whether the compounds are soluble or insoluble in water – Predicting the solubility of compounds in water is a fundamental aspect of chemistry, with far-reaching implications in various scientific disciplines and industrial applications. This article delves into the intricacies of solubility, exploring the factors that govern whether compounds dissolve in water or remain insoluble, providing a comprehensive understanding of this crucial phenomenon.
The chemical properties of compounds, intermolecular interactions, and empirical observations collectively determine their solubility in water. By unraveling the interplay between these factors, we gain the ability to predict the solubility of compounds, enabling us to design experiments, optimize processes, and develop new materials with tailored properties.
Solubility in Water
Solubility in water refers to the ability of a substance to dissolve in water, forming a homogeneous mixture. The extent of solubility depends on various factors, including the chemical properties of the substance and the temperature of the water.
Factors that affect solubility in water include:
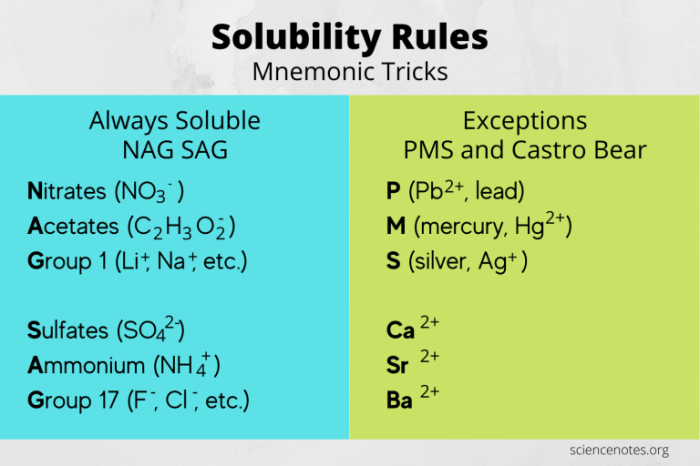
- Polarity:Polar substances, such as ionic compounds and polar covalent compounds, dissolve readily in water because they can form hydrogen bonds with water molecules.
- Molecular size:Smaller molecules tend to be more soluble in water than larger molecules.
- Temperature:In general, the solubility of most substances increases with increasing temperature.
Chemical Properties of Compounds
| Compound | Molecular Structure | Molecular Weight (g/mol) | Polarity |
|---|---|---|---|
| Sodium chloride (NaCl) | Ionic | 58.44 | Polar |
| Sucrose (C12H22O11) | Covalent | 342.30 | Polar |
| Hexane (C6H14) | Covalent | 86.18 | Nonpolar |
Polarity plays a crucial role in determining solubility. Polar compounds, like NaCl and sucrose, have a net positive or negative charge, allowing them to interact with the polar water molecules. Nonpolar compounds, like hexane, have no net charge and are therefore less soluble in water.
Intermolecular Interactions
Intermolecular interactions between water molecules and compounds contribute to solubility.
- Hydrogen bonding:Water molecules can form hydrogen bonds with polar compounds, creating strong attractive forces that promote solubility.
- Ion-dipole interactions:Ions, such as Na +and Cl –in NaCl, interact with the polar water molecules through ion-dipole interactions, leading to solubility.
- Van der Waals forces:Nonpolar compounds can interact with water molecules through weak Van der Waals forces, which contribute to their limited solubility.
Empirical Observations
Examples of compounds that are soluble in water include:
- Ionic compounds: NaCl, KCl
- Polar covalent compounds: Ethanol, sucrose
Examples of compounds that are insoluble in water include:
- Nonpolar covalent compounds: Hexane, oil
Predicting Solubility, Predict whether the compounds are soluble or insoluble in water
A decision tree can be used to predict the solubility of a compound in water:
- Is the compound polar?
- Yes:Proceed to step 2.
- No:The compound is likely insoluble in water.
- Is the compound an ionic compound?
- Yes:The compound is soluble in water.
- No:Proceed to step 3.
- Is the compound a polar covalent compound?
- Yes:The compound is likely soluble in water.
- No:The compound is likely insoluble in water.
FAQ Compilation: Predict Whether The Compounds Are Soluble Or Insoluble In Water
What is the significance of solubility in water?
Solubility in water plays a pivotal role in numerous scientific and industrial processes, including drug development, environmental remediation, and chemical synthesis. Understanding solubility allows us to optimize the delivery of drugs, design effective water treatment strategies, and develop new materials with desired properties.
How can I predict the solubility of a compound in water?
Predicting the solubility of a compound in water involves considering its chemical properties, intermolecular interactions, and empirical observations. By analyzing these factors, we can make informed predictions about the solubility of a given compound.
What are the limitations of predicting solubility?
Predicting solubility is not an exact science, and there are certain limitations to consider. Factors such as temperature, pressure, and the presence of other solutes can influence solubility, making it challenging to make precise predictions in all cases.